PD-L1 Companion Diagnostics and Scoring Systems
PD-L1 Approvals & Companion Diagnostics: January 2022 Update
This is an update to a post from June 2020, which contained a table of approved PD-L1 IHC companion diagnostics, the scoring systems they use, and the thresholds required for a positive result. Here is the action since then:
New PD-L1-based Approvals
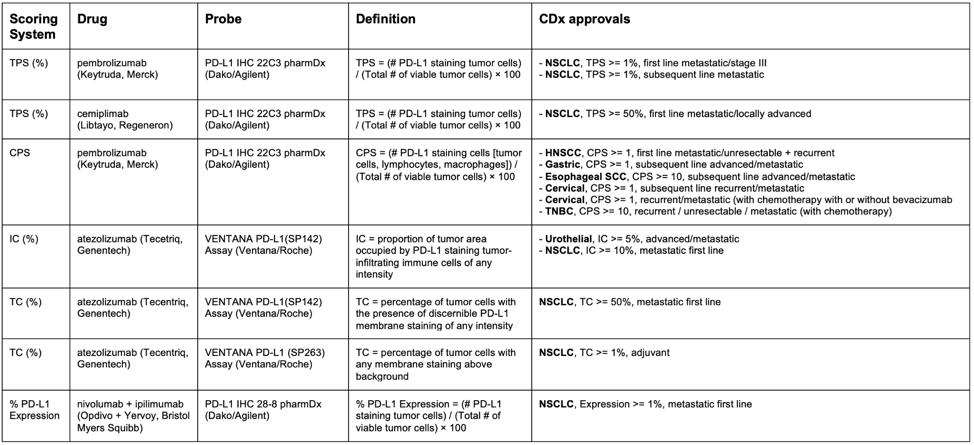
11/2020: pembrolizumab + chemotherapy, CPS >= 10, triple-negative breast cancer, recurrent/unresectable/metastatic (link)
2/2021: cemiplimab, TPS >= 50%, NSCLC, first line/metastatic/advanced (link)
10/2021: pembrolizumab + chemotherapy +/- bevacizumab, CPS >= 1, cervical carcinoma, recurrent/metastatic (link)
second pembrolizumab approval for cervical cancer with CPS >= 1
10/2021: atezolizumab, TC >= 1% NSCLC, adjuvant (link)
first PD-L1 based approval in the adjuvant setting
Rescinded PD-L1-based Approvals
8/2021: pembrolizumab approval for urothelial carcinoma (CPS >= 10) was updated so that it is no longer tied to PD-L1 expression (link)
8/2021: atezolizumab accelerated approval for TNBC (IC >= 1%) was withdrawn (link)
Here is the updated set of PD-L1 companion diagnostics and associated scoring systems, as of 1/17/2022:
GenomOncology’s precision oncology software continues to support the entry and interpretation of non-NGS results, including IHC (e.g. PD-L1, dMMR, HR, HER2), FISH, and karyotype/chromosome analysis.
[Original Post from 6/15/20]
FDA Approvals with PD-L1 Companion Diagnostics
Recently, FDA approved two new immunotherapies with PD-L1 based companion diagnostics, both for the first-line treatment of metastatic non-small cell lung cancer (NSCLC).
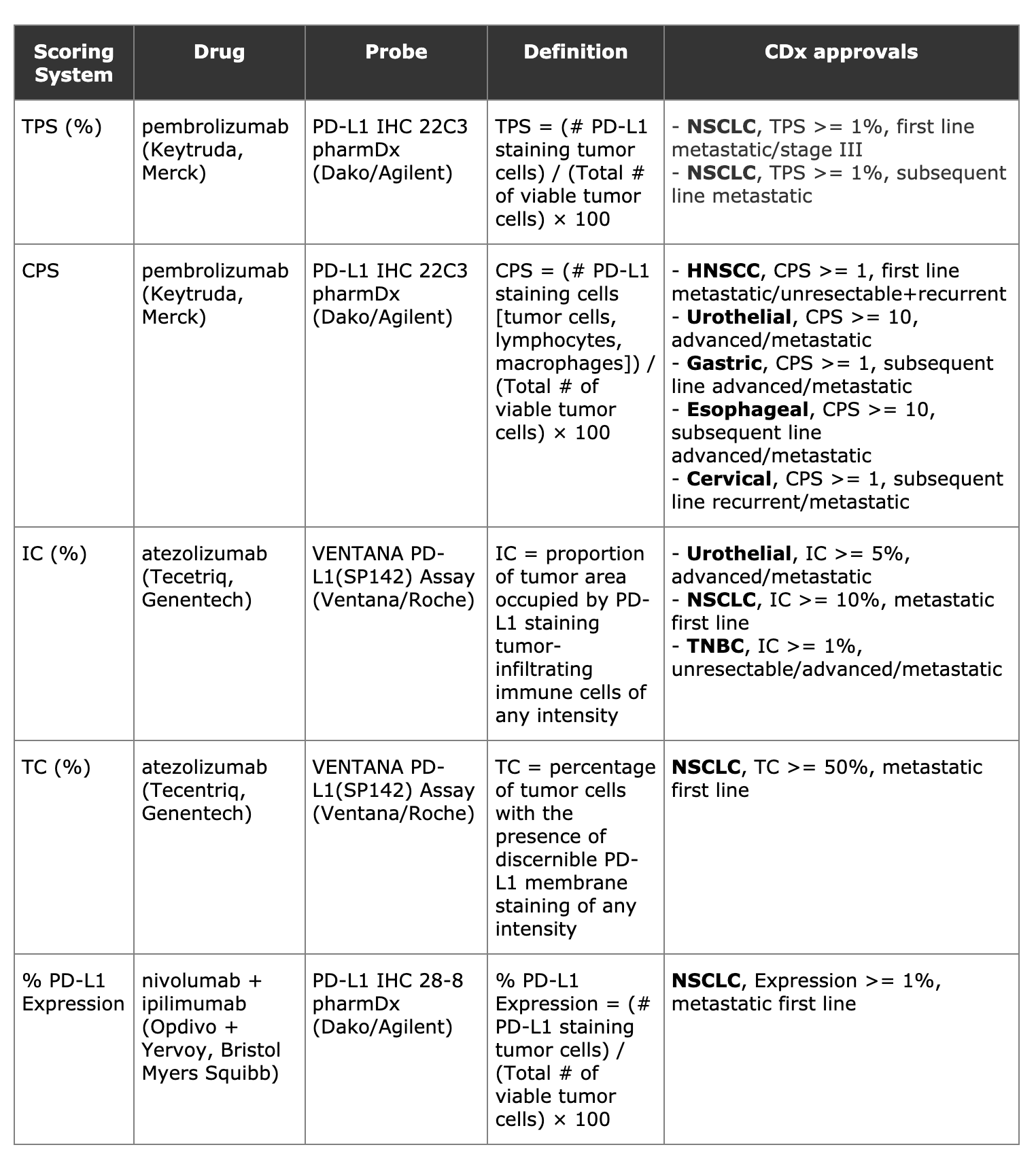
(1) Combination therapy nivolumab + ipilimumab, for PD-L1 expression >= 1%, and
(2) atezolizumab for high expression of PD-L1 (IC >= 10% or TC >= 50%)
Prior to these approvals, pembrolizumab was the only drug with a PD-L1 based companion diagnostic in NSCLC (based on TPS score >= 1%).
Previously, atezolizumab had PD-L1 based companion diagnostic for two other diseases -- urothelial carcinoma and triple negative breast cancer. Also, atezolizumab (in combination with chemotherapy), nivolumab, and nivolumab + ipilimumab had other approvals for NSCLC, but these were all regardless of PD-L1 expression levels.
The following table shows the new set of PD-L1 companion diagnostics and associated scoring systems as of 5/31/2020:
There are now three therapies with five different scoring systems for PD-L1 companion diagnostics. Three of the scoring systems are based on the percentage of tumor cells with PD-L1 staining (TPS, TC, % PD-L1 Expression), and seem to have equivalent definitions. IC is based on PD-L1 expression in tumor-infiltrating immune cells, while CPS (the only scoring system that is a number rather than a percentage) counts PD-L1 staining in both tumor cells and non-tumor cells.
Combined PD-L1 and NGS reporting
In my experience, most often PD-L1 results are reported and interpreted separately from NGS results. However, at GenomOncology, a growing number of our clients combine PD-L1 results and NGS results into a single report. This has a few advantages:
Simplicity. A single report with a unified interpretation.
Individual therapy indications involve both PD-L1 and NGS results. For example, both new approvals for NSCLC (atezolizumab and nivolumab + ipilimumab) require the tumor to be negative for ALK fusion and EGFR mutations, in addition to the companion diagnostic requirement for PD-L1.
Therapy selection depends on synthesizing PD-L1 and NGS results. Even when a patient is eligible for an immune checkpoint inhibitor according to the FDA label (e.g. PD-L1 expression + ALK negative + EGFR negative), if there is another oncogenic driver present such as BRAF V600E, ROS1/RET fusion, or MET exon 14 skipping, NCCN Guidelines recommend selecting a therapy based on that driver, instead of treating with the immune checkpoint inhibitor. A combined report can better convey this information.
PD-L1 Scores in Decision Support Software
In order to support our clients who report PD-L1 alongside NGS results, GenomOncology software handles PD-L1 scoring. The PD-L1 result for a given assay can be expressed as a single "alteration concept" that includes both the scoring system and the measured score result. For example:
PD-L1 Expression (TPS = 60%)
PD-L1 Expression (CPS = 3)
PD-L1 Expression (IC = 8%)
Our content team curates "therapy assertions" that indicate the range of valid scores. For example, this is a screenshot of the PD-L1 based pembrolizumab approval for HNSCC:
The GenomOncology Match Algorithm then ensures that any particular PD-L1 result matches properly to the appropriate therapies.
Please reach out to info@genomoncology.com with any questions.